Ocean acidification process
Known as the 'other carbon problem', ocean acidification is a critical consequence of rising carbon dioxide levels in the atmosphere. The ocean absorbs nearly 30% of carbon dioxide (CO₂) emissions from human activities, driving this process. As our oceans become increasingly acidic, marine ecosystems and the organisms that inhabit them face significant challenges.
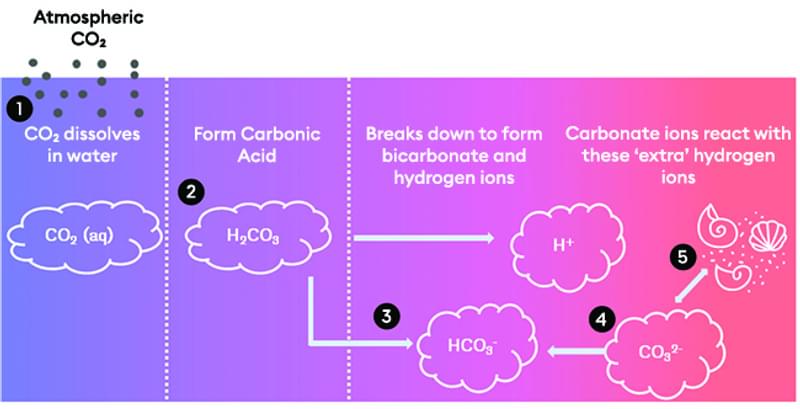
- Carbon dioxide (CO2) from the atmosphere dissolves in the ocean, creating aqueous carbon dioxide (CO2 (aq)).
- This dissolved CO2 reacts with water (H2O) to form carbonic acid (H2CO3).
- Carbonic acid then breaks down into bicarbonate (HCO3-) and hydrogen ions (H+). The increase in hydrogen ions makes the ocean more acidic. As more carbon dioxide is absorbed, the ocean becomes increasingly acidic due to the rising number of hydrogen ions.
- Natural processes like the weathering of rocks help counteract this increase in acidity. For example, chalk (CaCO3) from eroding cliffs is added to the ocean over time. Carbonate ions (CO32-) from the chalk react with the hydrogen ions, forming more bicarbonate ions (HCO3-). This process, called buffering, reduces the number of carbonate and hydrogen ions in the ocean.
- However, this buffering comes at a cost. Marine organisms that build shells or skeletons from carbonate have fewer carbonate ions available. This change places stress on these species, forcing them to use extra energy to build their structures, leaving less energy for growth and reproduction. In severe cases, the ocean's acidity may reach a level where shells or skeletons start to dissolve to buffer the ocean's pH, endangering these organisms even further.

Geography | Ages 14-16
Frozen Oceans
This Frozen Oceans education resource includes two data case studies that introduce students to ocean acidification and sea ice thickness. The core of each case study are data sets from real expeditions.

Science | Ages 11-14
Frozen Oceans
The Frozen Oceans Science resources introduce working scientifically concepts and skills to 11-14-year-olds through enquiry-based lessons which replicate work done by field scientists in the Arctic.

Science | Ages 14-16
Frozen Oceans
This Frozen Oceans unit outlines the research carried out by the Catlin Arctic Surveys and can be used in teaching the carbon cycle, ocean acidification and its impact on the Arctic ecosystem.
